Ultrafast Reaction Mechanism in Perovskite Bases Photocatalytic C-C Coupling
Jan. 21, 2020
Photoexcited perovskite nanocrystals (NCs) can initiate photo-redox reactions by reducing and oxidization various substrates on ultrafast timescale. The lifetime of the excited nanocrystals limits the subsequent reactive pathways.
Scientific Achievement
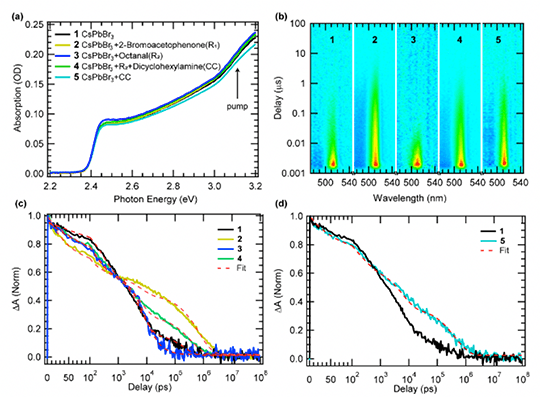
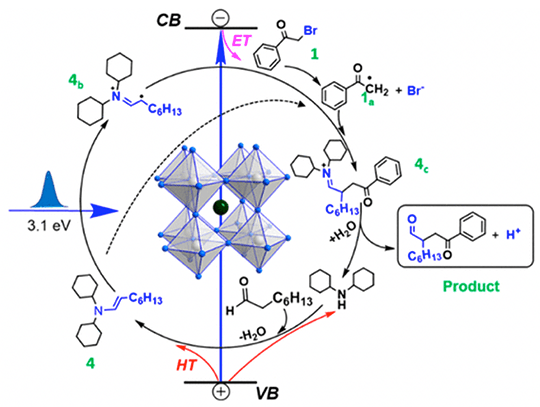
We observed an ultrafast electron transfer (~50 ps) from CsPbBr3 NCs to 2-bromoacetophenone and hole transfer (~70 ps) to an in-situ formed enamine or cocatalyst, resulting in a charge separated state with ~0.8 μs lifetime that allows photogenerated radical intermediates to couple.
Significance and Impact
Carbon-carbon (C-C) bond formation is a highly desirable chemical transformation. New types of catalyst that can activate small molecules are highly sought after. NC photo redox catalyst offers advantages over molecular homogenous catalyst.
Research Details
- Transient absorption (TA) measurements with and without presence of substrates and co-catalyst
- Photo-excited NCs can both reduce and oxidize the reagents forming long-lived radical species